Shivkripa Healthcare – Global Exporter of Generic Medicines, Pharma Products & Specialty Drugs from India
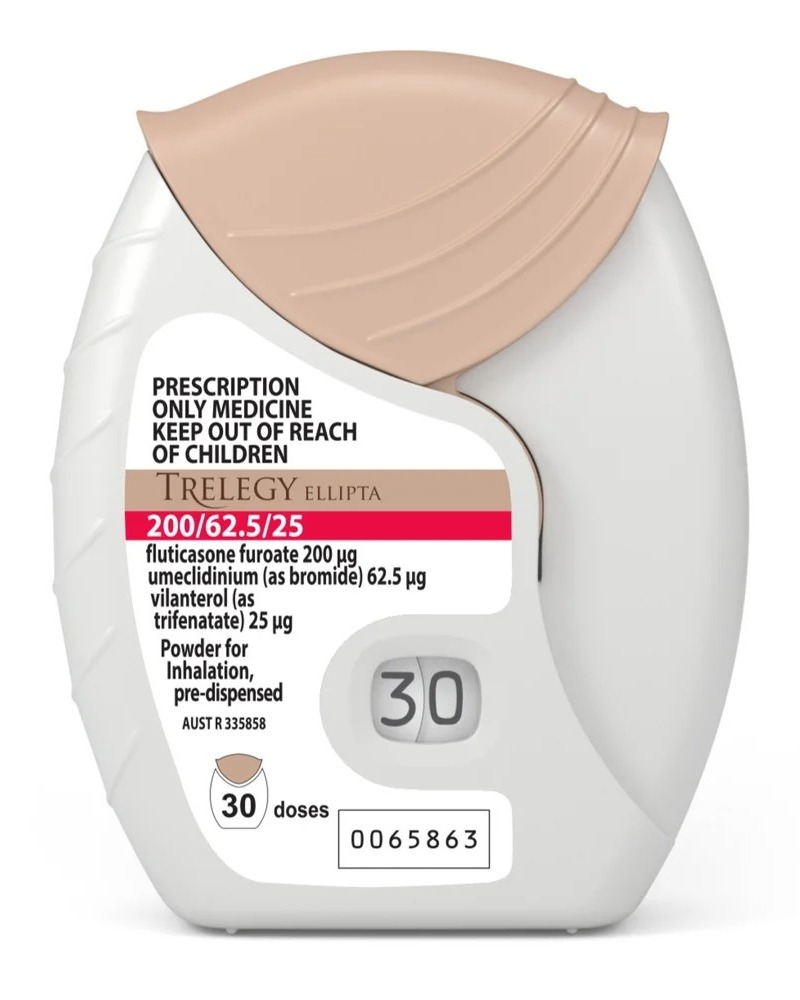
Looking for a reliable source to import Trelegy Ellipta Inhalers in Canberra? Shivkripa Healthcare supplies globally trusted inhalation therapy solutions for chronic respiratory conditions, catering to pharmacies, hospitals, and medical distributors in over 30+ countries.
Trelegy Ellipta is a once-daily inhaler used for managing COPD (Chronic Obstructive Pulmonary Disease) and asthma. Each inhaler contains a combination of fluticasone furoate, umeclidinium, and vilanterol, offering bronchodilation and inflammation control in a convenient format. Our export stock is sourced from WHO-GMP certified units, ensuring safe and consistent quality.
We serve buyers across Canberra, as well as international markets like London, Washington, Canberra, Singapore, and Manila. Whether you are a hospital buyer, pharmacy chain, or licensed reseller, we provide export-ready packaging, regulatory documentation, and timely dispatch.
We support both low and bulk order volumes, offering CIF pricing, transparent paperwork, and proactive customer assistance for smooth import clearance.
Need Trelegy Ellipta Inhalers in Canberra? Get assured quality, fast international delivery, and end-to-end support with Shivkripa Healthcare.
Visit www.medicinesdropshipping.com or connect via WhatsApp: Click here to chat
Frequently Asked Questions
1. Do you export Trelegy Ellipta to hospital and pharmacy buyers?
Yes, we supply Trelegy to licensed hospitals, pharmacy distributors, and large-volume medical buyers across global markets.
2. Is Trelegy Ellipta available for both COPD and asthma patients?
Yes, depending on the country’s approved usage. It is commonly prescribed for long-term maintenance in COPD and asthma.
3. Can I get required documentation for customs in Canberra?
Absolutely. We provide COA, MSDS, invoice, packing list, and necessary regulatory export documents.